Nereda biowatch studying the visual language of aerobic granular sludge
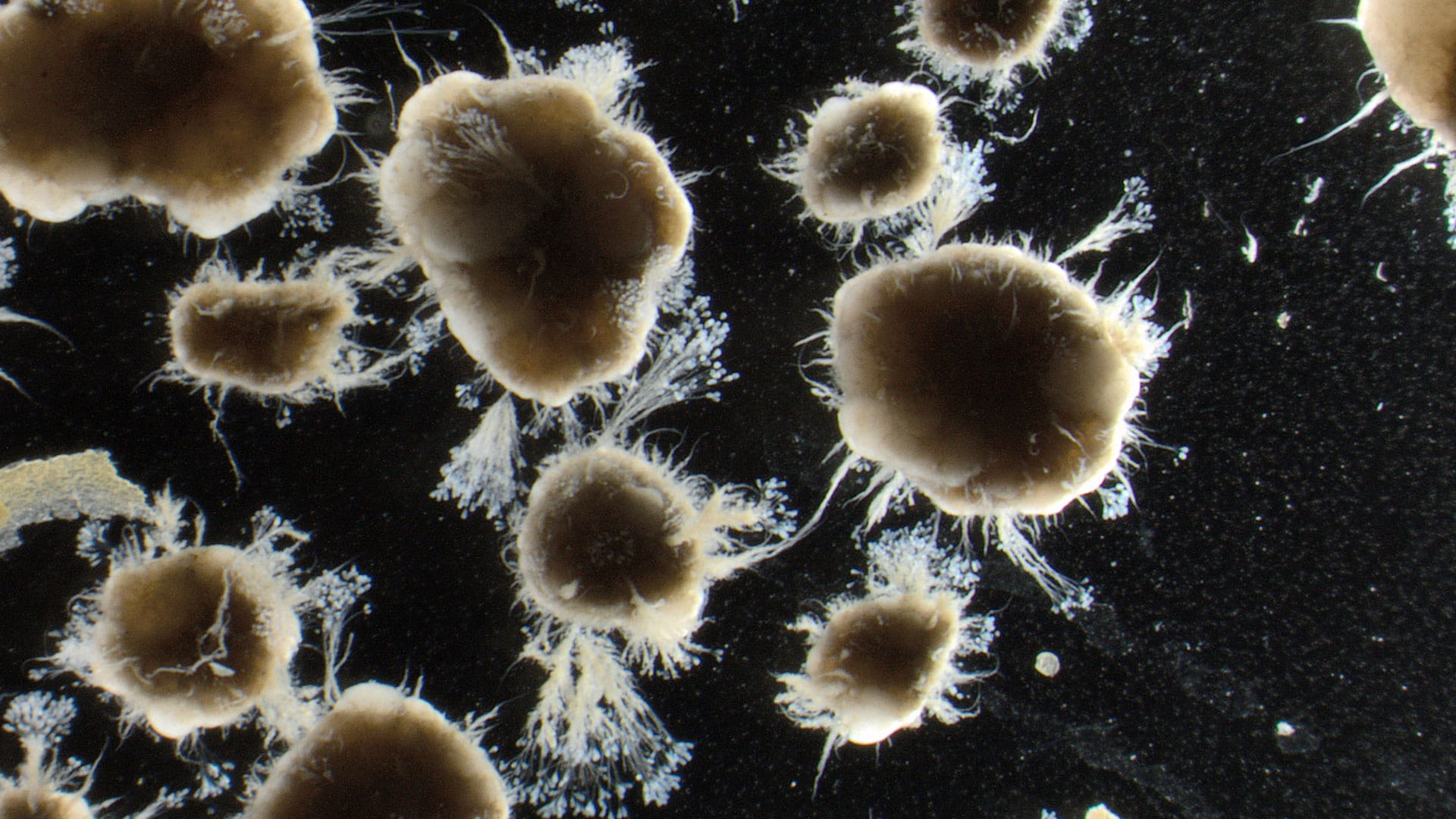
Microscopy in Watewater treatment systems
First of all, let’s talk about method. Scientists have been studying bacteria through microscopes since Antonie van Leeuwenhoek first observed them in 1674. Fittingly enough for the topic of this blog, he was studying a drop of lake water at the time. His notes on these experiments have become quite famous:"I now saw very plainly that these were little eels, or worms, lying all huddled up together and wriggling just as if you saw, with the naked eye, a whole tubful of little eels and water, with the eels squirming among one another; and the whole water seemed to be alive with these multifarious animalcules.
This was for me, among all the marvels that I have discovered in nature, the most marvelous of all; and I must say, for my part, that no more pleasant sight has every yet come before my eyes that these many thousand of living creatures seen all alive in a little drop of water, moving among one another, each several creature having its own proper motion.”[1]
Since van Leeuwenhoek spotted his little animalcules, many improvements have been made in both microscope technology and in the visual study of microbes. However, this technology was only extensively reported on as useful for wastewater treatment systems around 1975, resulting in a manual for microscopic sludge investigation published in 1983[2] (followed by an updated and expanded version in 2000[3]) by professor Eikelboom. He reported that the quality of flocculent sludge could be studied visually, by examining which type of microbes are present in and around the floc.

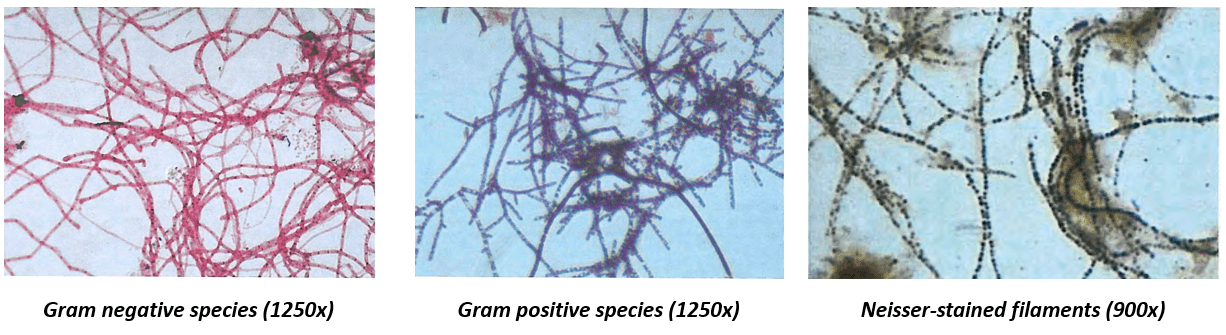
To see individual bacteria, which have a diameter of 1-10 micrometer – a hundred to a thousand times smaller than a human hair - a light microscope with a zoom of 100-1000x is usually used. To view bacteria at such high magnifications, a very small drop of sludge (about 0.005 mL) is pressed between two glass slides[4]. Additional techniques can be used to help identify the bacteria, such as chemical staining protocols such as Gram or Neisser staining, or even fluorescent genetic probes. These chemicals color only certain types of microbe. Some images of stained species with different methods (from the Eikelboom (2000) book) are shown below. The captions show the type of staining and the magnification of the image.
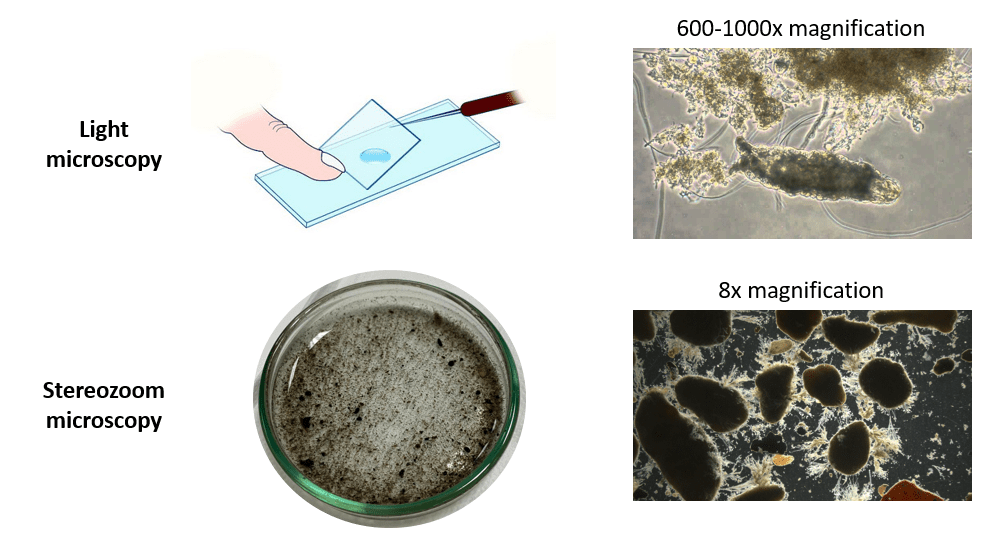
Therefore, for Nereda Biowatch we use a different method. Instead of a light microscope, we use a stereozoom microscope. We apply lower magnification, only 8-50x, and use 3D samples, not flattened ones. The added benefit is that we can use larger sample sizes, about 5-10 mL instead of 0.005 mL, which makes the analysis more representative. The figure below shows the difference in analysis sample and presents typical images for both analyses.[5]
Biofilms
So, what is a biofilm? A biofilm is an aggregate of cells growing together in a matrix of extracellular polymers (usually sugar polymers and proteins). An extremely simplified schematic of this is shown below. Nereda granules consist of bacteria and other microbes growing together in such a biofilm.

Slime layers are biofilms, so are activated sludge flocs, and so are compact and dense sludge granules. Biofilms can take on many shapes, depending on the available food, the environmental conditions, and the types of organisms.
Morphology of aerobic granular sludge
Growing microbes in biofilms is useful for wastewater treatment. Because the bacteria grow together in dense structures, high levels of dry solids can be attained. A carrier is sometimes used to facilitate the attachment of microbes, such as in IFAS systems. In other cases, like with Nereda, no carrier is used to make the biofilms grow. Instead, selective pressures are applied that give a benefit to microbes that coagulate together. Other principles that are necessary for granulation are also applied, such as a proper feeding regime. These conditions together stimulate the growth of granular biofilms.
The basic principle of Nereda Biowatch is that these pressures and conditions, and how effectively they are applied, can be recognized by the outward appearance, or morphology, of the granules and flocs. The Nereda cycle has all the elements to grow smooth and dense granules. By understanding these elements, microscopic analysis can help to find problems with granulation.
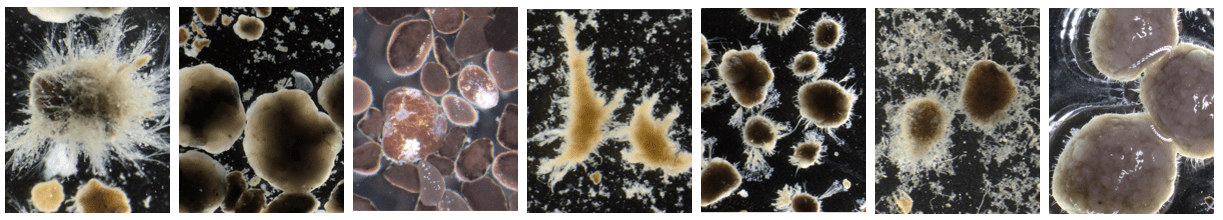
The image below shows an example of this. The manner in which COD is taken up during the cycle, mostly aerobically or mostly anaerobically, results in different granule morphologies. The arrow colors in the figure show that from left to right, the general image of the sludge progressively deteriorates.

Another use is in monitoring: the analysis can serve as an early detection analysis. Changes in the process can often be noticed visually in the sludge morphology before the effects of potential issues are seen in effluent quality. Practical examples of this are that Biowatch has helped in troubleshooting to recognize when too many solids were recirculated or when the feeding phase had to be extended.
So, Nereda Biowatch can be used for monitoring and troubleshooting, and also provides a new and very visual way to “read” the sludge in the reactor. This different method of microscopy compared to studying the individual microbes may not show all the different wriggling multifarious animalcules that have compelled scientists from van Leeuwenhoek on, but they do show us many fascinating sludge structures and provide us with intriguing insights on biofilm morphology in Nereda systems.
Please get in touch by clicking the button below.
[2] Eikelboom, D. H., & Van Buijsen, H. J. J. (1983). Microscopic sludge investigation manual.
[3] Eikelboom, D. H. (2000). Process control of activated sludge plants by microscopic investigation. IWA publishing.
[4] Eikelboom, D. H. (2000). Process control of activated sludge plants by microscopic investigation. IWA publishing.
[5] Light microscopy slide image source: https://coordinatedscience1.wordpress.com/lessons/unit-4-cells/4-3-more-microscope-practice/